JAMB - Chemistry (2011 - No. 9)
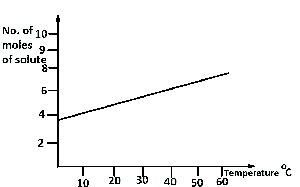
From the diagram above, find the amount of solute deposited when 200 cm\(^3\) of the solution is cooled from 55°C to 40°C.
0.10 mole
0.20mole
0.01 mole
0.02 mole
Explanation
From the diagram,
55°C = 7 moles; 40°C = 6 moles.
Amount of solute deposited = 7 - 6 = 1 mole.
1000 cm\(^3\) = 1 mole
200 cm\(^3\) = x
x = \(\frac{200 \times 1}{1000}\)
= 0.20 mole.
Comments (0)


