JAMB - Chemistry (2006 - No. 45)
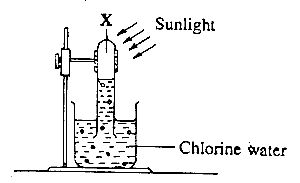
In the diagram above, gas X is
hydrogen
chlorine
oxygen
hydrogen chloride
Explanation
When chlorine water (a solution of chlorine gas in water) is exposed to sunlight, chlorine reacts with water molecules to form hypochlorous acid (HClO), which then decomposes into hydrochloric acid (HCl) and oxygen gas (O\(_2\)).
- Initial Reaction: Cl₂ + H₂O ⇌ HClO + HCl
-
Decomposition of Hypochlorous Acid: HClO → HCl + [O] (nascent oxygen)
-
Formation of Oxygen Gas: [O] + [O] → O₂
-
Overall Reaction: 2Cl₂ + 2H₂O → 4HCl + O₂
N.B: Nascent oxygen refers to the atomic form of oxygen (represented as [O]) that is highly reactive and unstable.
Comments (0)


