JAMB - Chemistry (1995 - No. 44)
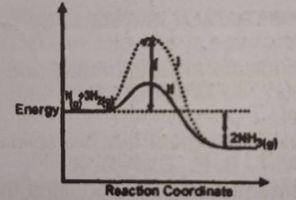
It can be deduced that the rate of the reaction?
for path l is higher than path ll
for path ll is higher than path l
is the same for both paths at all temperatures
depends on the values of both x and y at all pressures
Explanation
A catalyst does affect the rate of a chemical reaction, generally by increasing it, by providing an alternative reaction pathway with a lower activation energy.
Path II is actually a catalysed reaction while path I is uncatalysed. Thus, the rate of reaction in path II is higher than path I.
Comments (0)


