JAMB - Chemistry (1993 - No. 41)
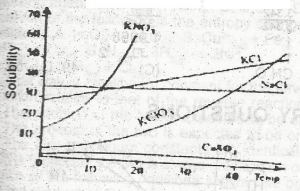
For which salt in the graph above does the solubility increase most rapidly with rise in temperature?
CaSO4
KNO3
NaCl
KCl
Explanation
For many solids that are dissolved in water or any liquid, the solubility increases with a temperature rise. With an increase in kinetic energy, the solvent molecules effectively break apart the solute molecules that are held together by strong intermolecular attractions, and thereby solubility is increased.
Comments (0)


