JAMB - Chemistry (1989 - No. 44)
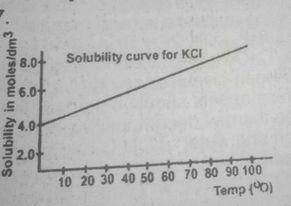
If in the graph above 1 dm 3 of a saturated solution of KCL is cooled from 80°C, the mass of crystals deposited will be
[K = 39, Cl = 35.5]
7.45 g
14.90 g
74.50 g
149.00 g
Explanation
Trace the graph to the y-axis to obtain the solubility of the salt at 80°C and at the point where the straight line touches the y-axis, the solubility of the salt at these respective temperatures is 4mol\dm3 and 6mol\dm3
solubility of salt that will crystallize out = 6- 4 = 2mol\dm3
therefore mass of salt deposited = solubility x molar mass of salt
2 x 74.5 = 149.00g
Comments (0)


