JAMB - Chemistry (1986 - No. 37)
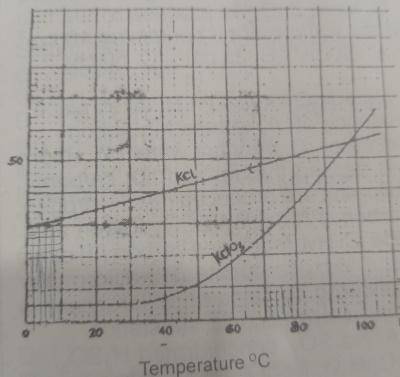
In the solubility curve above, water at 98\(^0\)C is saturated with KCl and KCIO\(_3\). What is the percentage of KCl impurity in the crystals formed when the solution is cooled to 30\(^0\)C?
51.5
45.5
34.5
26.5
Explanation
From the graph, when you try to extrapolate the temperature to the solubility value of both KCl and KCIO\(_3\), we'll arrive at the following data:
| KCl | KClO\(_3\) | |
|
@ 98\(^0\)C |
55 | 55 |
|
@ 30\(^0\)C |
37 | 5 |
| Difference | 18 | 50 |
Total weight dissolved in 100g of water = 18 + 50 = 68g
% KCl impurity = \(\frac{weight of KCl}{Total weight}\times 100\)
= \(\frac{18}{68}\times 100\)
= 26.47
= 26.5%
Comments (0)


