JAMB - Chemistry (1985 - No. 31)
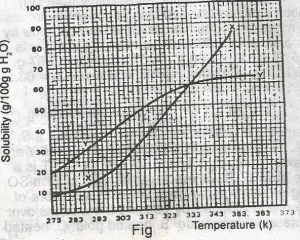
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If the molar mass of X is 36 g, the number of moles of X dissolved at 343 K is
0.2 moles
0.7 moles
1.5 moles
2.0 moles
3.0 moles
Explanation
The molar mass of x = 36g. At 343k, 72g of x dissolved
Molarity of x = \(\frac{72}{36}\) = 2.00moles.
Comments (0)


