JAMB - Chemistry (1985 - No. 30)
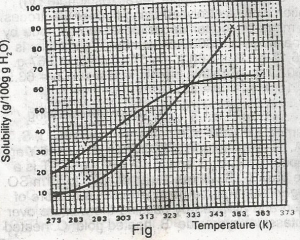
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If 80 g each of X and Y are taken up in 100 g of water at 353 K we shall have
only 10g of X undissolved
only 16 g of Y undissolved
10 g of X and 16 g of Y undissolved
all X and Y dissolved
all X and Y undissolved
Explanation
For salt X, from the graph, 80g of X dissolves by 349K, so by 353K, all 80g of X is dissolved totally in 100g of water.
For salt Y, from the graph, 64g of Y dissolves by 353K, so (80-64)g of Y is left undissolved. This is 16g of Y left undissolved at that temperature.
Comments (0)


