JAMB - Chemistry (1980 - No. 40)
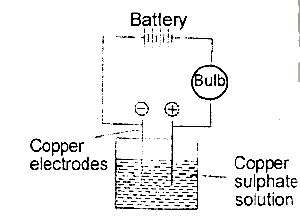
Copper sulphate solution was electrolysed using the apparatus show above. Which of the following changes are observed?
The bulb lights and copper is deposited at both electrodes
the bulb lights and copper is deposited at the anode and disappears from the cathode
the bulb lights and copper is deposited at the cathode and oxygen liberated at the anode
the bulb lights and copper is deposited at the cathode and disappears from the anode
the bulb lights but no additional changes are observed
Explanation
Copper electrodes are chemically related to CuSO4
Anode: \(Cu^{2+}\) → Cu + 2e
Cathode: \(Cu^{2+}\) + 2e- → Cu
The anode gets thinner, the cathode gets thicker, and work is done in the external circuit to light the bulb.
Comments (0)


