JAMB - Chemistry (1980 - No. 38)
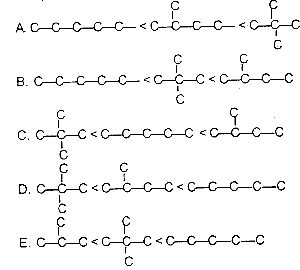
Which of the following is the correct order in the above diagram increasing boiling point, of the isomeric \(C_{5}H_{12}\) compounds?
A
B
C
D
E
Explanation
The boiling points of each of the compounds:
Pentane - 36.1°C ; 2- methylbutane - 27.8°C ; dimethylpropane - 10°C.
Therefore, the order of boiling point in ascending order is Dimethylpropane < 2-methylbutane < pentane.
Comments (0)


