JAMB - Chemistry (1978 - No. 45)
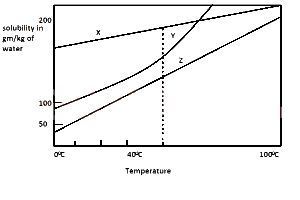
Consider the solubility curves of three salts X, Y and Z given in the diagram. If each solution of the salt contains 200 g, and is heated to 100\(^o\)C, which solution or solutions will deposit 100 g of the solute when suddenly cooled to 0\(^o\)C?
X only
Y only
Z only
X and Z
Y and Z
Explanation
If you study the curve very well, looking at the y-axis where we have the solubility, you will discover that Salt X exceeds the 100g/kg mark while Salt Y and do not. This simply implies that solutions Y and Z will deposit 100 g of the solute when suddenly cooled to 0\(^o\)C, since they didn't exceed the solubility value of 100g, abinitio.
Comments (0)


